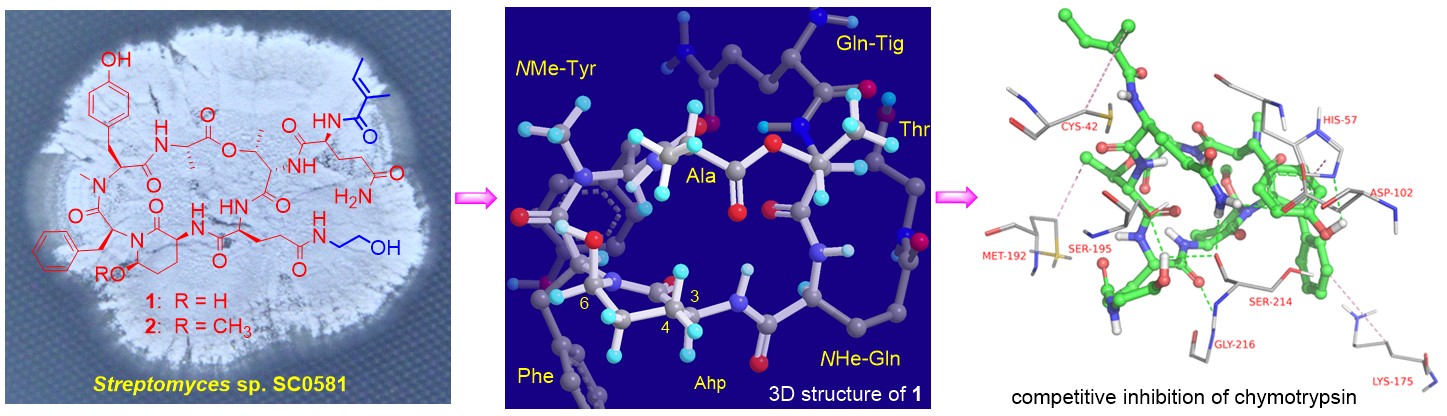
(1) Chymotrypsin Inhibitory Cyclodepsipeptides Produced by a Soil-Derived Streptomyces Strain
Four new cyclodepsipeptides, dinghupeptins A-D (1-4), possessing a rare N5-(2-hydroxylethyl)glutamine moiety, were isolated from the cultures of the soil-derived Streptomycessp. SC0581. Their structures were elucidated by spectroscopic and advanced Marfey's amino acid analysis and their 3D structures were established by theoretical conformational analysis. Compounds 1 and 2, containing an Ahp unit, displayed selective inhibition of chymotrypsin with IC50 values of 2.1 and 1.1 μM, respectively. Enzyme kinetic analysis and molecular docking experiments revealed they are competitive inhibitors binding to the active site of chymotrypsin.
Ahp containing cyclodepsipeptides have attracted great interest from researchers in organic chemistry, medicinal chemistry, and chemical biology because many of them display potent inhibition of serine proteases with various selectivity characteristics. Our study provided a new method for 3D structure elucidation of this family of compounds and new insights into the SARs of Ahp containing cyclodepsipeptides in inhibition of serine proteases. The study was published in Journal of Natural Products (2018, 81: 1928–1936) and was selected as an “editor’s choice” article.
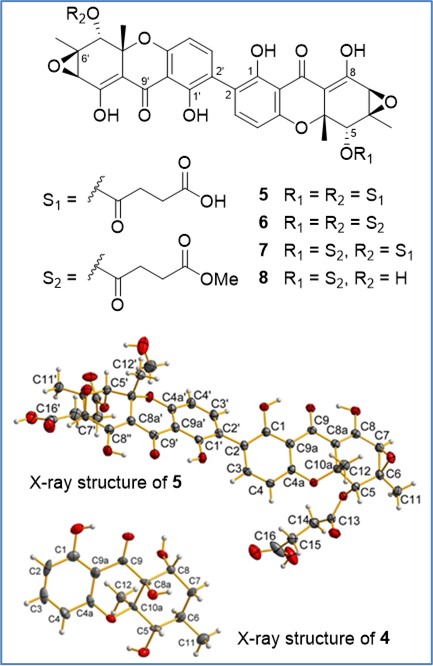
Eight new polyhydroxanthones, penicixanthones A−H (1−8), were isolated from solid cultures of Penicillium purpurogenum SC0070. Their structures were elucidated by extensive spectroscopic ananlysis, X-ray single-crystal diffraction, and theoretical computations of ECD spectra. Penicixanthone G (6) displayed the strongest antibacterial activity (MIC: 0.4 μg/mL) against both Staphylococcus aureus and the methicillin-resistant strain MRSA. Penicixanthone D (4) is distinct from other penicixanthones in stereochemistry, and its biosynthetic mechanism was proposed based on theoretical simulations for the reaction pathway of C-10a epimerization. The study was published in Journal of Natural Products (2020, 83, 1480–1487).
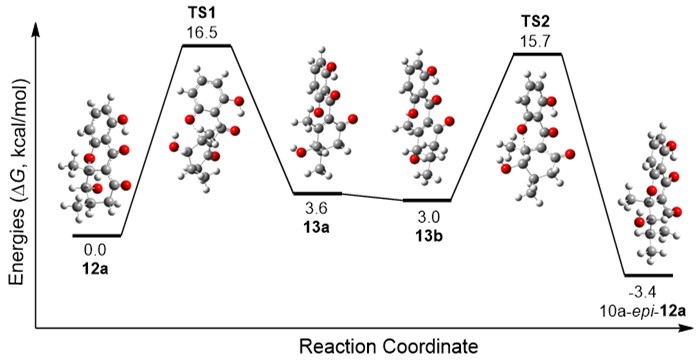
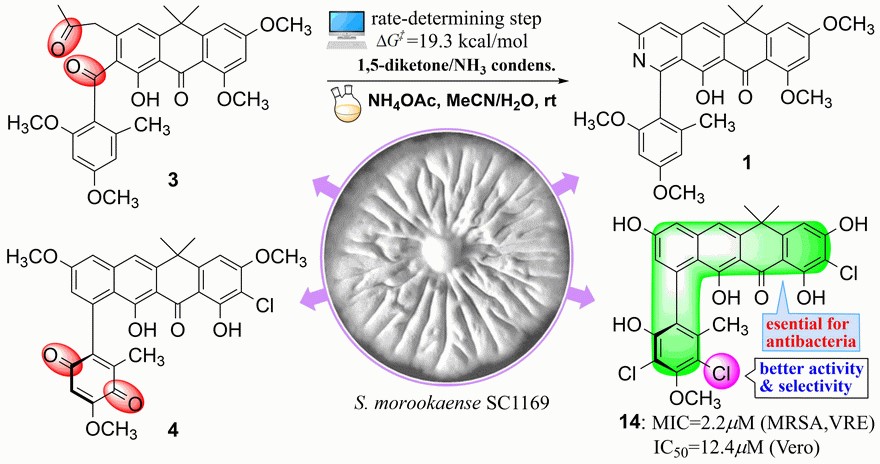
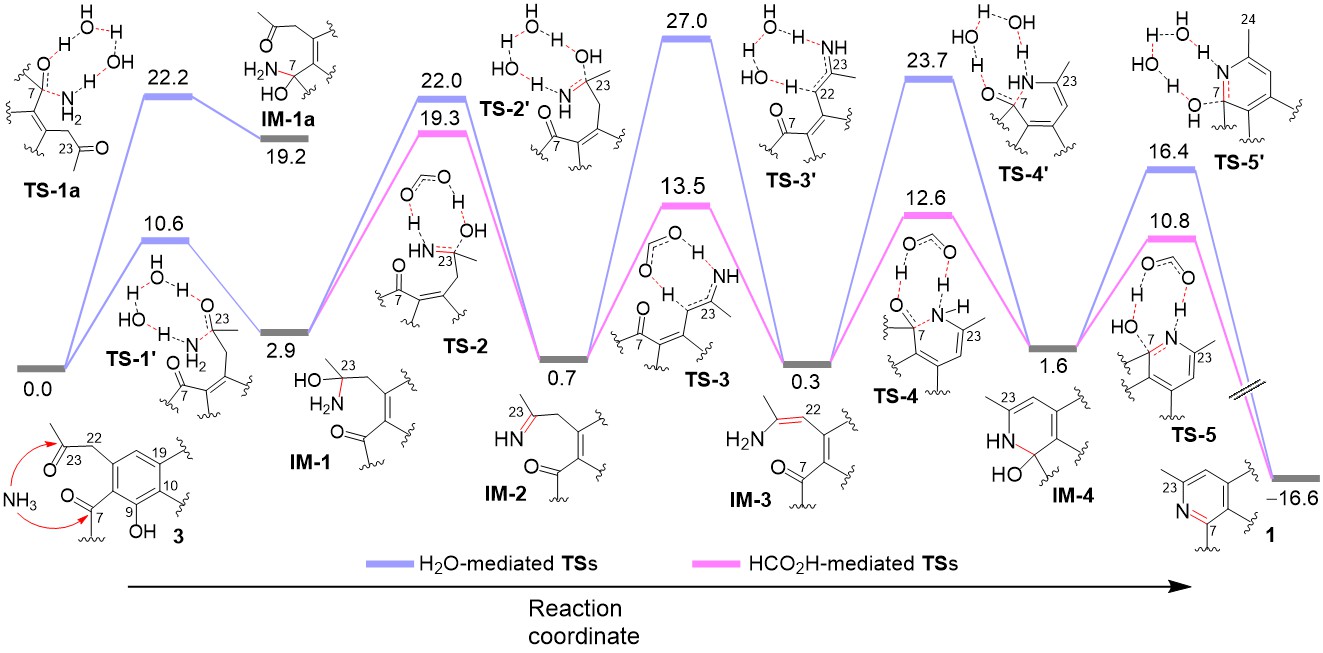
Formicapyridine-type racemates, streptovertidines A (1) and B (2), a 7,24-seco-fasamycin, streptovertidione (3), and the fasamycin-type streptovertimycins I–T (4-15), together with 13 known fasamycin congeners (16-28), were isolated from soil-derived Streptomyces morookaense SC1169. Their structures were elucidated by extensive spectroscopic analysis and theoretical computations of ECD spectra. Theoretical simulations of reaction paths and chemical reactions for conversion of 3 to 1 were carried out and supported that the pyridine ring formation in formicapyridines proceeds non-enzymatically via 1,5-dicarbonyl condensation with ammonia.The fasamycin-type compounds 5, 812, 14, and 15 exhibited activity against the drug-resistant bacteria MRSA and VRE (MIC: 1.25–10.0 μg/mL). All isolates, except 3, 4, 10, and 24, displayed cytotoxicity against at least one of the human carcinoma A549, HeLa, HepG2, and MCF-7 cells (IC50 < 10.0 μM), of which some were also cytotoxic to the non-cancerous Vero cells. Taken together, the activity data demonstrated that the fasamycin-type compounds were more selective to the tested bacteria over the mammalian cells. The study was published in Journal of Natural Products (2021, 84: 1806–1815)。

(4) Specialized metabolites in Casuarina equisetifolia
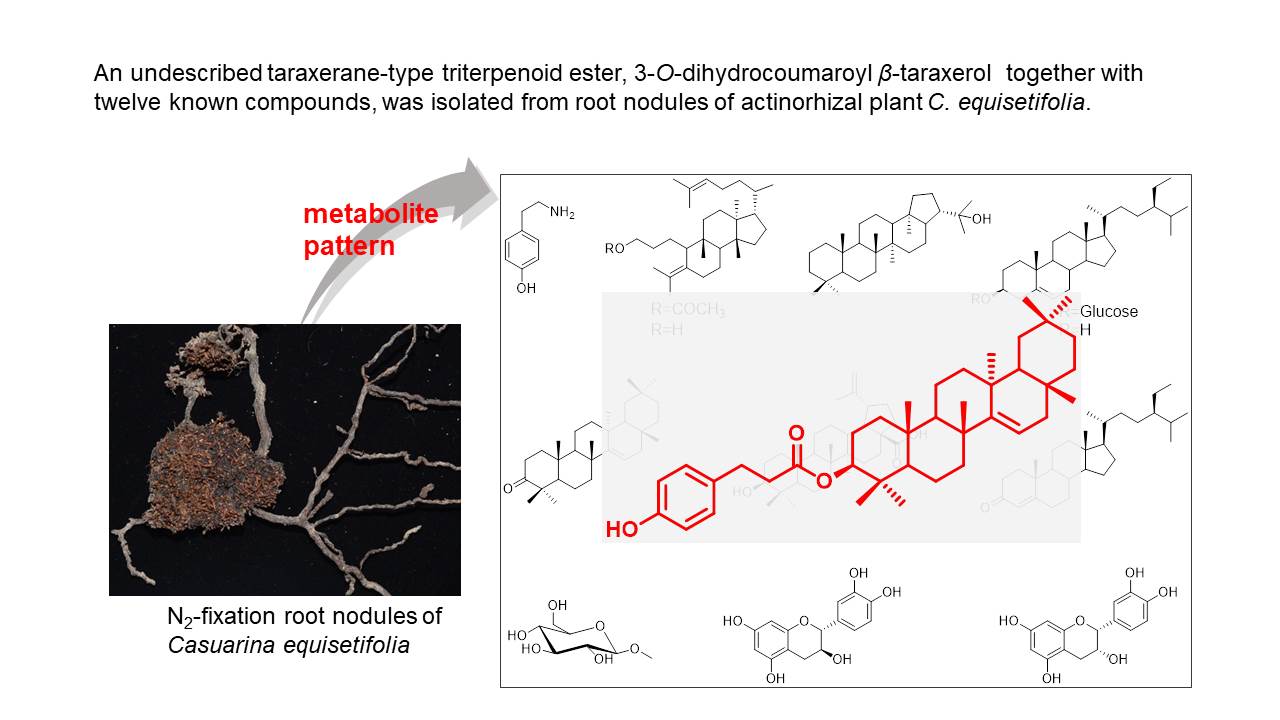
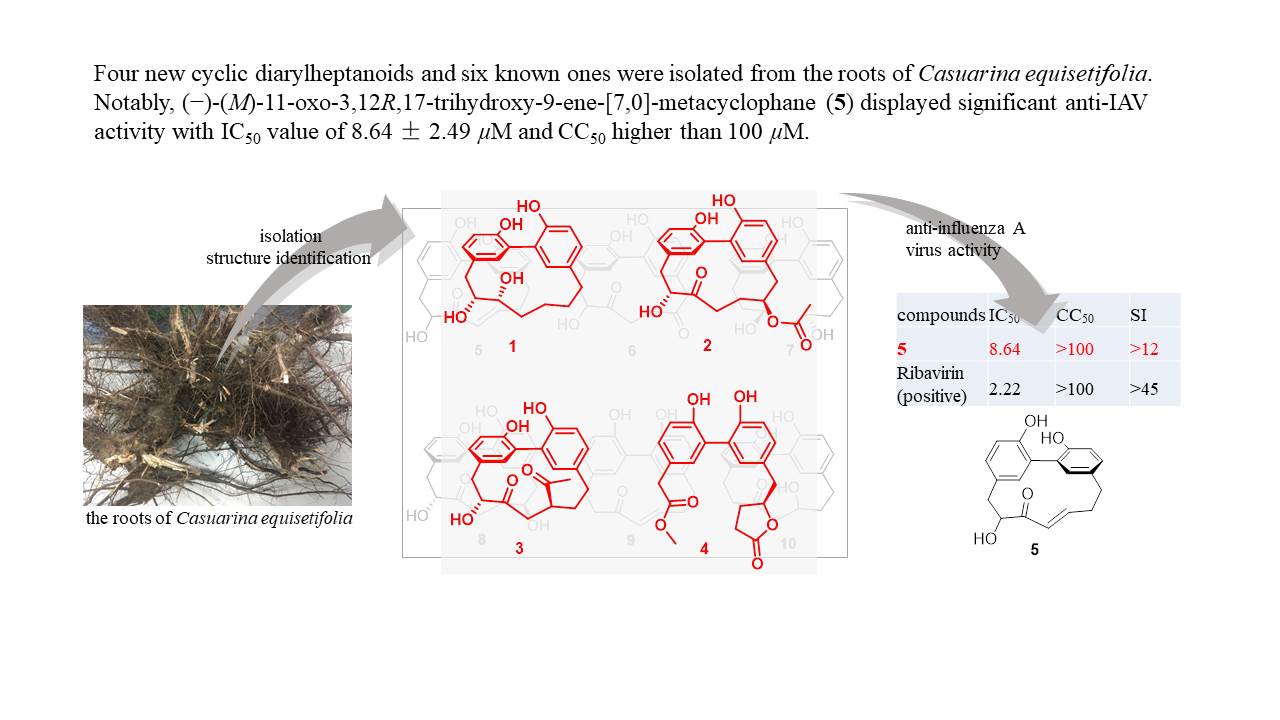
Casuarina equisetifolia L. (Casuarinaceae) is an actinorhizal plant, and forms N2-fixing nodules. A metabolomic approach was applied to root nodules of mature C. equisetifolia trees, leading to the identification of an undescribed taraxerane-type triterpenoid ester, 3-O-dihydrocoumaroyl β-taraxerol, along with twelve known compounds. An abundant component was tyramine in mature nodules. Tyramine specifically and abundantly accumulated in mature nitrogen-fixing nodules compared to senescent nodules, stems, leaves, and seeds. Four new cyclic diarylheptanoids, casuarinols A-C (1-3) and casuarinolide A (4), together with six known ones (5-10), were isolated from the roots of C. equisetifolia. Casuarinol C (3) is a novel cyclic diarylheptanoid-aldehyde adduct. Casuarinolide A (4) represents the first structure of a seco-cyclic diarylheptanoid. (−)-(M)-11-oxo-3,12R,17-trihydroxy-9-ene- [7,0]-metacyclophane (5) displayed significant anti-influenza A virus activity against A/WSN/33 (H1N1) with IC50 value of 8.64 ± 2.49 µM and CC50 higher than 100 µM. The results about root nodules was published in Phytochemistry (2021, 186, 112724), while the results about roots was published in Journal of Natural Products (2022, doi: 10.1021/acs.jnatprod.2c00335).
(5) Nutritional Health Guided Design and Processing Key Technologies for Subtropical Fruits and Vegetables and Their Industrialization
The project was granted the Second Class Prize of National Science and Technology Progress Award in 2020, and South China Botanical Garden (SCBG), CAS was the third completion institution. SCBG participated in the study of chemical characterization of phenolics from subtropical characteristic fruits and vegetables and contributed 17 SCI papers, and 16 of which the corresponding author are Prof. Haihui Xie.
Longan, litchi, bitter gourd, and kudzu vine root and so on are typical subtropical fruits and vegetables (SFVs) with nutritional health effect (NHE). Firstly, aiming at key scientific and technological problems in SFVs, such as unclear active substance basis for NHE and action mechanism, lack of theory and technology of deep processing, and few high additional value products, we created efficient separation and characterization technologies for polysaccharides, polyphenols, and triterpenes, clarified the chemical structures of major active components (MACs), solved the bottleneck problems of unclear MACs in the design and formulation of healthy foods. Secondly, we revealed the molecular mechanism of macromolecule active polysaccharides in SFVs modulating intestinal immunity and improving learning and memory, established key design and processing technologies of functional foods with polysaccharides as MACs, and created a series of functional foods with the effects of modulation immune system, improving memory and sleep as well as specific foods for intestinal mucosal repair. Thirdly, we ascertained the structure- and dose-effective relationships of liver protection, glucose and lipid metabolism of small molecule active polyphenols and triterpenes in SFVs, established key design and formulation technologies of healthy foods on the basis of high efficient preparation and utilization of active polyphenols and triterpenes, and developed functional foods with liver protection and antioxidant effects as well as specific foods for the diabetes. The project contained 31 authorized invention patents, 71 SCI papers, 2 First Class Prizes of Guangdong STP Award, and 1 silver prize of China Patent, 28 new products, and 7 approvals of healthy or specific foods, and made important contributions to leading the nutritional health guided deep-processing development of SFVs.
